The global number of COVID-19 cases and deaths has fallen for the sixth week in a row, marking a sharp downward trend in the pandemic. So much so, in fact, that many countries are planning a return to something resembling “normality” by June or July.
Read more
The U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the third vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The EUA allows the Janssen COVID-19 Vaccine to be distributed in the U.S for use in individuals 18 years of age and older.
Read more
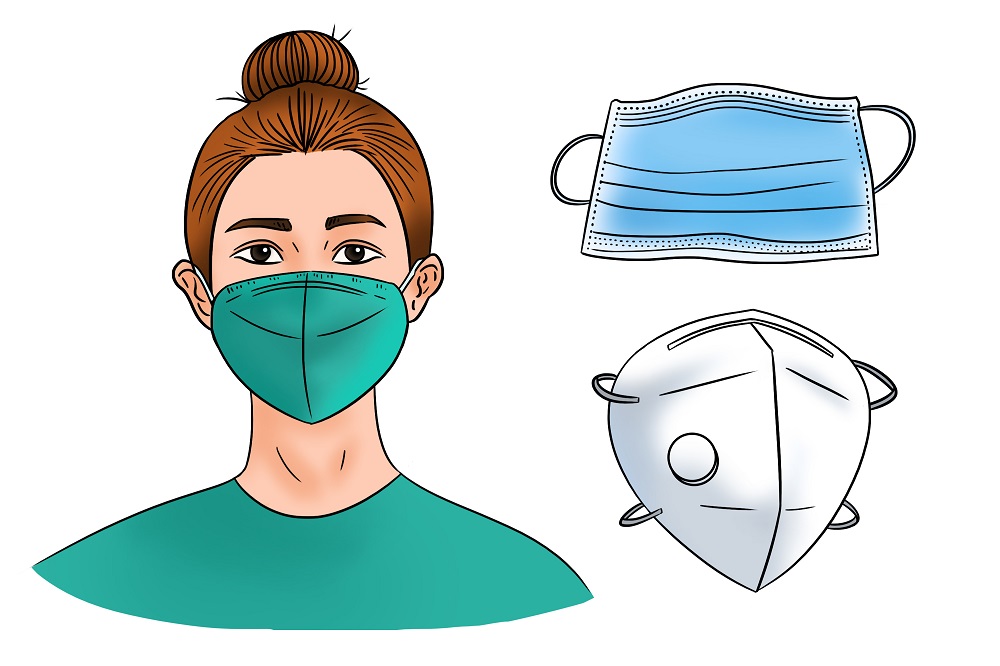
Correct and consistent mask use is a critical step everyone can take to prevent getting and spreading COVID-19. Masks work best when everyone wears them, but not all masks provide the same protection. When choosing a mask, look at how well it fits, how well it filters the air, and how many layers it has.
Read more
The United States currently has the safest vaccine supply in its history. Vaccine Safety is a vital part of the nation’s response to the COVID-19 pandemic. As vaccines are developed and become available, the public’s knowledge of their safety, both initially and during extended use, is an important part of a successful vaccination effort.
Read more

The Corona vaccine teaches our immune system how to recognize and fight the virus that causes COVID-19. Sometimes this process can cause symptoms, such as fever. These symptoms are normal and are a sign that the body is building protection against the virus that causes COVID-19. So vaccines are needed to protect us...
Read more
A new variant strain of SARS-CoV-2 that contains a series of mutations has been described in the United Kingdom (UK) and become highly prevalent in London and southeast England. Based on these mutations, this variant strain has been predicted to potentially be more rapidly transmissible than other circulating strains of SARS-CoV-2.
Read more
People with a history of severe allergic reactions should not get the Pfizer / Bioanth vaccine. This recommendation includes carriers of people who have been allergic to drugs, food or vaccines in the past. Those who have been shown to be allergic to the Pfizer vaccine have been treated and recovered. Allergic reactions are usually rare, but can occur with certain vaccines, such as the annual flu shot.
Read more
The seeds of doubt are being sown before a vaccine has even been approved. It is time to prepare the public for what could be a significant hurdle for any COVID-19 vaccine.
Read more
Throughout this pandemic, you've likely heard terms like Phase 1-2a trial and FDA approval, but what do these really mean—and at what point do they happen?
A vaccine would allow the body to safely develop an immune response to COVID-19 that could prevent or control infection.
it takes time to develop safe and effective vaccines – usually five to ten years on average. Despite promising reports about potential coronavirus vaccines being developed worldwide, it could take an estimated 12-18 months to develop one.
Read more

Occupational health programs and public health officials making decisions about return to work for healthcare personnel (HCP) with confirmed SARS-CoV-2 infection, or who have suspected SARS-CoV-2 infection (e.g., developed symptoms of COVID-19) but were never tested for SARS-CoV-2.
Read more











Recent Comments